Characterization of Occult Metastatic Disease in Pancreatic Cancer
Hepatic occult metastases represent the major obstacle to achieve cure for patients with localized pancreatic cancer, yet remarkably little is known about this disease state. This stems from two major challenges. First, “normal” liver tissue, which is required to study this biologic system in human patients, has traditionally been excluded from biobanking programs. Second, the experimental platforms required to study ultrarare populations and their interactions have only recently been developed, and still require significant technical expertise. Accordingly, no treatments exist to specifically target occult metastatic disease, and thus long-term survival is achieved in fewer than 5% of patients who undergo potentially “curative” surgery.
A central theme of our research focuses on characterizing occult metastatic disease and the microenvironment supporting or suppressing metastatic outgrowth, with the long-term goal of devising novel treatment strategies specific to this clinical challenge. A key step forward requires the accurate quantification of occult metastatic cells within the otherwise normal host organ, data which do not currently exist in human patients.
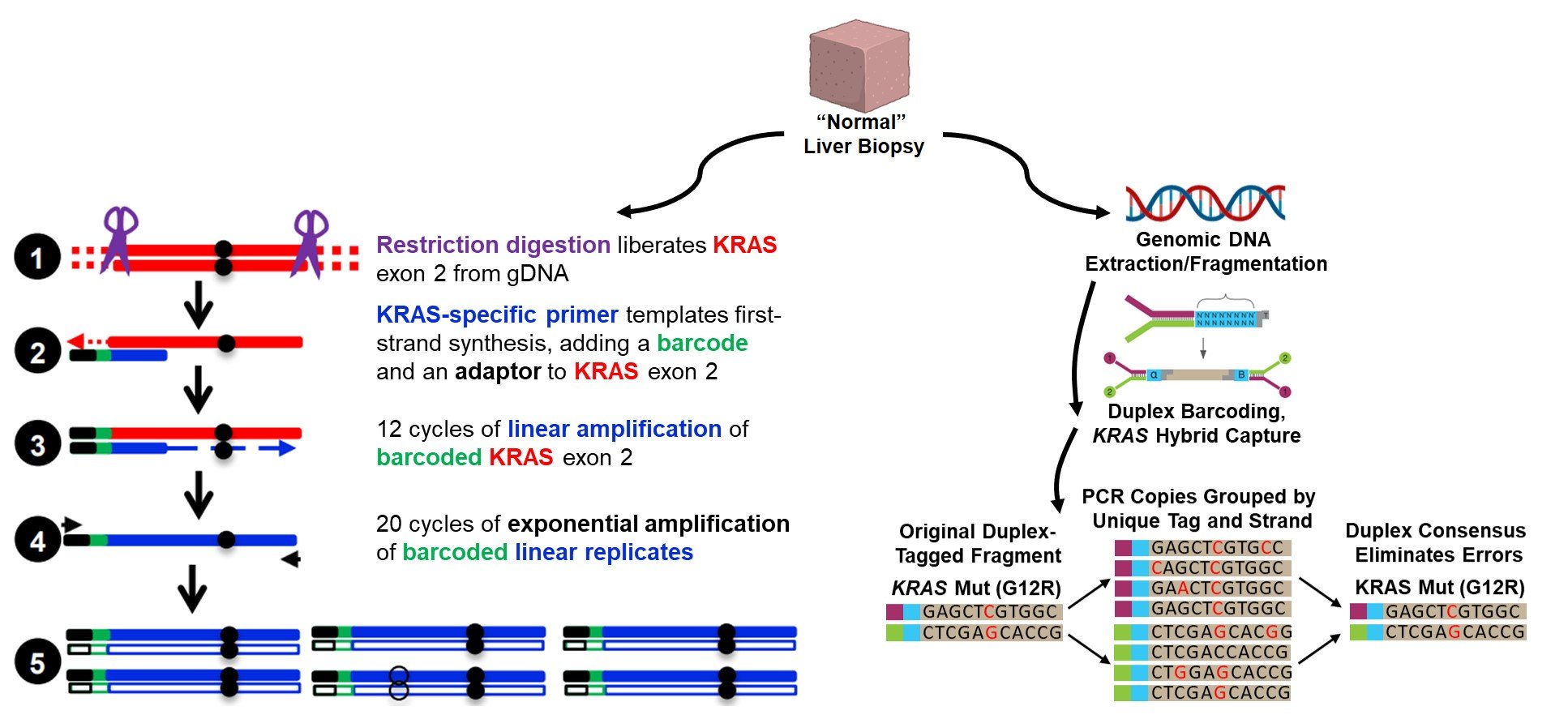
Leveraging the genomic uniformity of pancreatic adenocarcinoma (95% harbor a point mutation in the G12 or G13 codon of KRAS exon 2), we have adapted error-corrected next-generation sequencing methods (e.g. duplex sequencing, maximum depth sequencing) to accurately quantify occult metastatic cells vastly outnumbered within the parenchyma of an otherwise “normal” host organ. This represents a critical first step towards more detailed phenotypic analysis of the occult metastatic process.